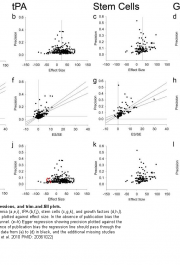
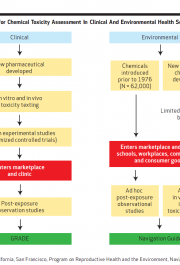
The US National Toxicology Programme has announced the release date of its case-study protocols, scheduled April 9, to test its planned approach for introducing systematic review techniques into its processes for evaluating chemical safety. There is an article about it in this month’s Environmental Health Perspectives. And there is this, from the NTP website (note the download […]