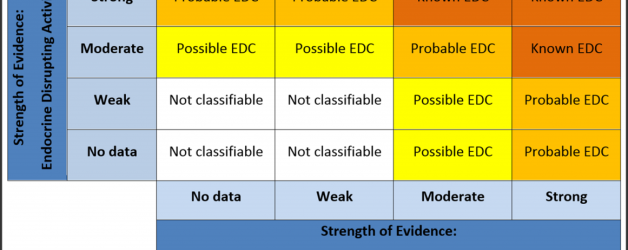

We are contributing authors to a new publication which, we hope, presents a coherent framework for using existing evidence to classify chemicals as EDCs. The devil will be in the detail, of course, but one of the major contributions the paper makes is its clear and structured guidance for weighing evidence (or as we prefer to call it, “systematic review and integration of evidence”) of endocrine disruption.
Some of the discussion around identifying EDCs for regulatory purposes centres on a requirement for “firm” evidence. “Firm” is of course an ambiguous term: everyone involved in the process could probably agree that we need “firm” evidence before triggering regulatory controls, yet everyone could have completely different ideas as to what counts as “firm” evidence.
The framework paper addresses this by laying out a process for agreeing on just how firm the evidence is that a chemical is an EDC, as a means of establishing the state of scientific knowledge in a process separate from the political decision which needs to be made as to how firm evidence needs to be before a regulator will respond to it.
This should be very important for reducing disagreement, because it sets a standard for what counts as “firm”, or “very firm” or “quite firm”, which is transparent and a reproducible judgement between reviewers, and therefore puts a brake on the scientisation of the politics of regulating EDCs – because the scientific process of classifying the evidence is insulated from the political process of deciding when and how to respond to it.
